Checklist Preparing For Clinical Research Visits At Home
Research demands meticulous attention to detail where mistakes have serious consequences. This Checklist Preparing For Clinical Research Visits At Home ensures systematic excellence in study conduct, from protocol adherence to regulatory compliance. Build quality into every action, protecting patients and data integrity.
Initial Planning Phase (2-4 Weeks Before)
□ Review study protocol thoroughly
□ Identify all visit requirements and procedures
□ Create visit-specific checklist from protocol
□ Coordinate with study team members
□ Review budget and resource allocation
□ Assess staffing needs for visit
□ Plan visit schedule and timeline
□ Identify potential challenges or barriers
Participant Preparation (1-2 Weeks Before)
□ Contact participant to schedule visit
□ Send pre-visit instructions and requirements
□ Confirm participant’s home address and directions
□ Verify participant has necessary space for visit
□ Discuss any special accommodations needed
□ Remind about fasting or other preparations
□ Confirm participant availability for full visit duration
□ Arrange for caregiver presence if required
Regulatory Preparation
□ Ensure current IRB approval
□ Verify informed consent is up-to-date
□ Check for any protocol amendments
□ Review HIPAA and privacy requirements
□ Prepare confidentiality measures
□ Update regulatory binders
□ Confirm insurance and liability coverage
□ Review institutional policies for home visits
Supply Inventory & Organization
□ Create master supply list from protocol
□ Inventory all supplies and equipment
□ Order any missing items
□ Organize supplies by procedure
□ Label all containers and bags
□ Prepare backup supplies
□ Check expiration dates
□ Create supply tracking sheet
Equipment Preparation & Testing
□ Test all electronic equipment
□ Calibrate measurement devices
□ Charge all batteries
□ Prepare backup equipment
□ Clean and sanitize equipment
□ Pack equipment securely
□ Create equipment checklist
□ Prepare troubleshooting guide
Documentation Preparation
□ Print all necessary forms (multiple copies)
□ Prepare electronic data capture access
□ Create source document templates
□ Organize forms by visit procedure
□ Prepare participant handouts
□ Include extra blank forms
□ Waterproof important documents
□ Create documentation tracking log
Team Coordination
□ Assign team member responsibilities
□ Schedule pre-visit team meeting
□ Review visit procedures with team
□ Discuss contingency plans
□ Confirm team member availability
□ Share emergency contact information
□ Plan communication during visit
□ Designate point person for issues
Logistics Planning
□ Plan travel route and alternatives
□ Arrange transportation/parking
□ Check weather forecast
□ Prepare for different scenarios
□ Plan visit timeline with buffers
□ Coordinate specimen pickup/delivery
□ Arrange for interpreter if needed
□ Plan for pet management
Risk Management Planning
□ Conduct risk assessment for home visit
□ Develop safety protocols
□ Create emergency response plan
□ Identify nearest medical facilities
□ Prepare incident report forms
□ Review liability insurance
□ Plan for adverse events
□ Establish check-in procedures
Week of Visit Preparation
□ Confirm visit with participant (48 hours before)
□ Check all equipment functionality
□ Review participant’s recent data
□ Pack all materials systematically
□ Prepare day-of contact list
□ Review weather and traffic
□ Confirm team member readiness
□ Send reminder to participant (24 hours before)
Day Before Visit
□ Final equipment check
□ Charge all devices overnight
□ Pack vehicle with supplies
□ Review protocol one more time
□ Prepare paperwork packet
□ Check emergency supplies
□ Confirm with team members
□ Set multiple alarms
Day of Visit Final Checks
□ Review checklist one final time
□ Confirm participant is ready
□ Check traffic and weather
□ Ensure all supplies are loaded
□ Verify documentation is complete
□ Test communication devices
□ Brief team on day’s plan
□ Conduct final safety check
How the Checklist Preparing For Clinical Research Visits At Home works
Specify research type, phase, and regulatory framework. Input your role and responsibilities. The AI creates a comprehensive checklist preparing for clinical research visits at home with temporal organization and role-based filtering. Track compliance items and maintain inspection readiness.
Research violations trigger investigations, halt studies, and destroy careers. Most are preventable oversights, not willful misconduct. This checklist ensures systematic compliance that protects everyone involved. It’s how professional research organizations maintain quality.
Meet the smartest dictation for auto-formatted and ready-to-send text
WriteVoice turns your voice into clean, punctuated text that works in any app. Create and ship faster without typing. Your first step was Checklist Preparing For Clinical Research Visits At Home; your next step is instant dictation with WriteVoice.
A blazing-fast voice dictation
Press a hotkey and talk. WriteVoice inserts accurate, formatted text into any app, no context switching
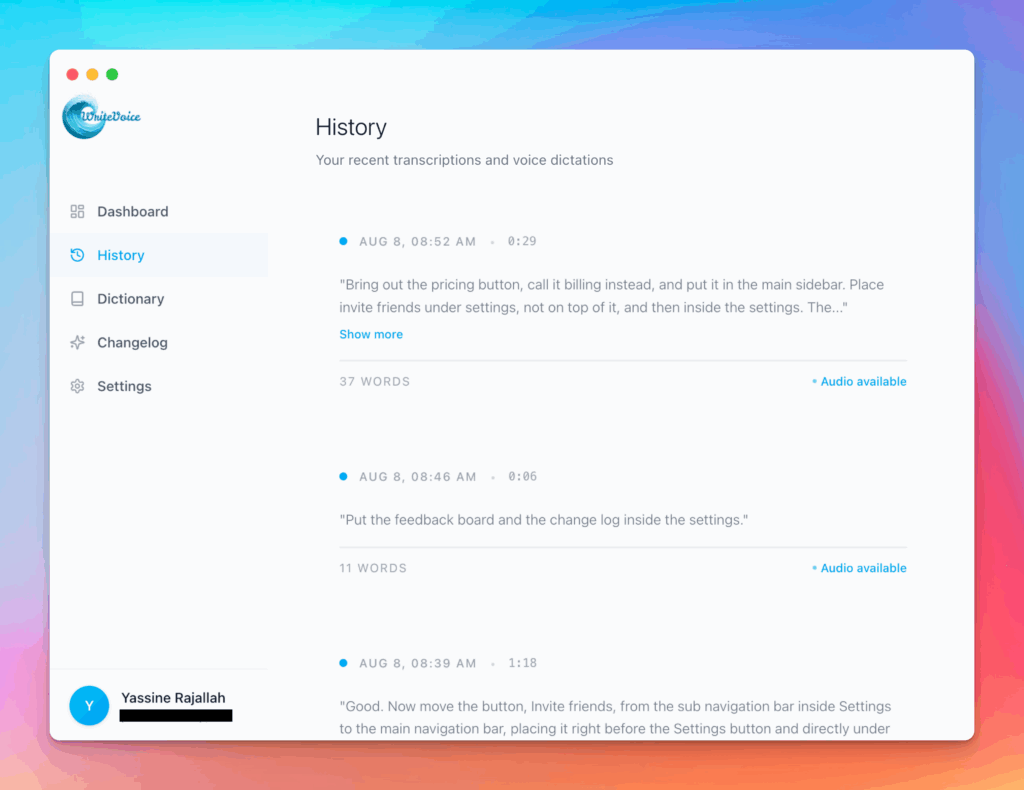
Works in any app
Press one hotkey and speak; your words appear as clean, punctuated text in Slack, Gmail, Docs, Jira, Notion, and VS Code—no context switching, just speed with writevoice
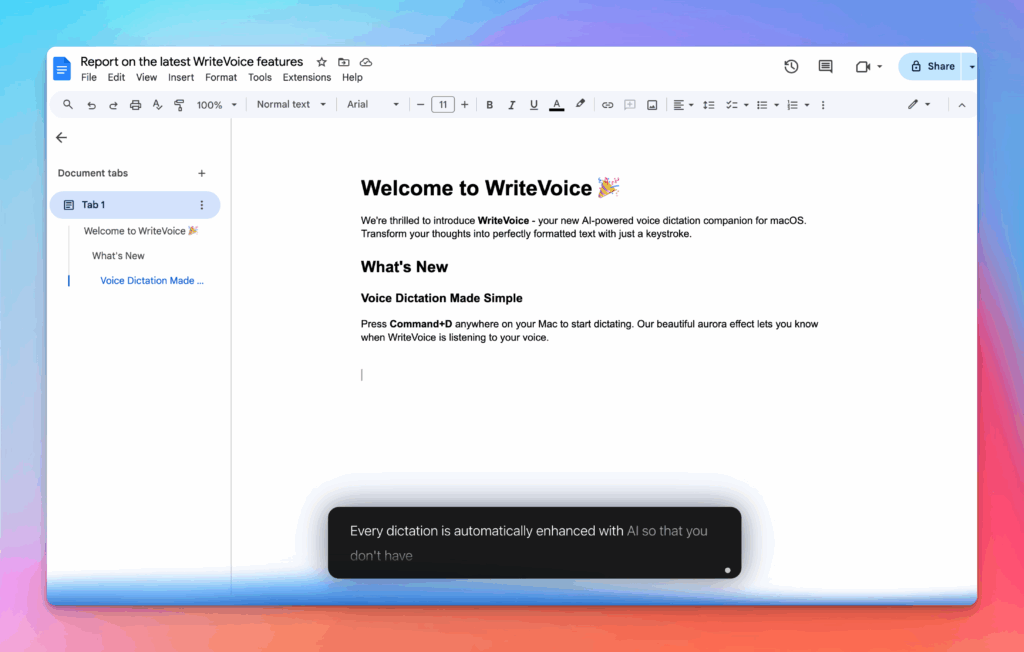
Accurate, multilingual, and smart
97%+ recognition, smart punctuation, and 99+ languages so your ideas land first try, built for teams and pros.
Private by default
Zero retention, audio and text are discarded instantly, with on-device controls so you can dictate sensitive work confidently.

